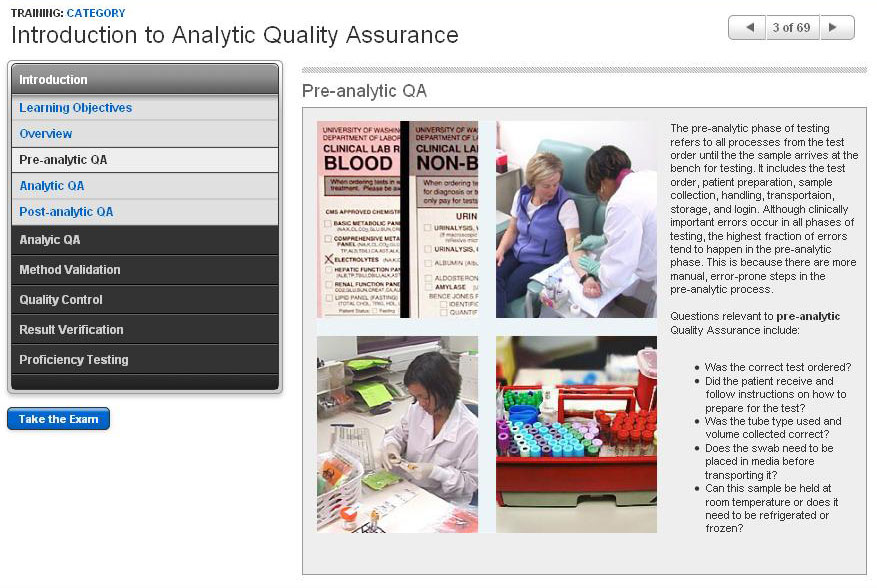
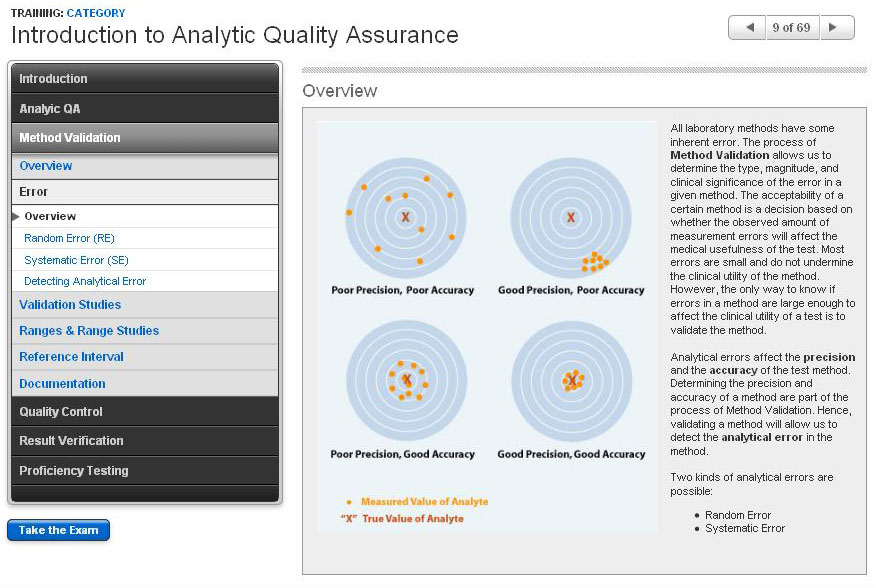
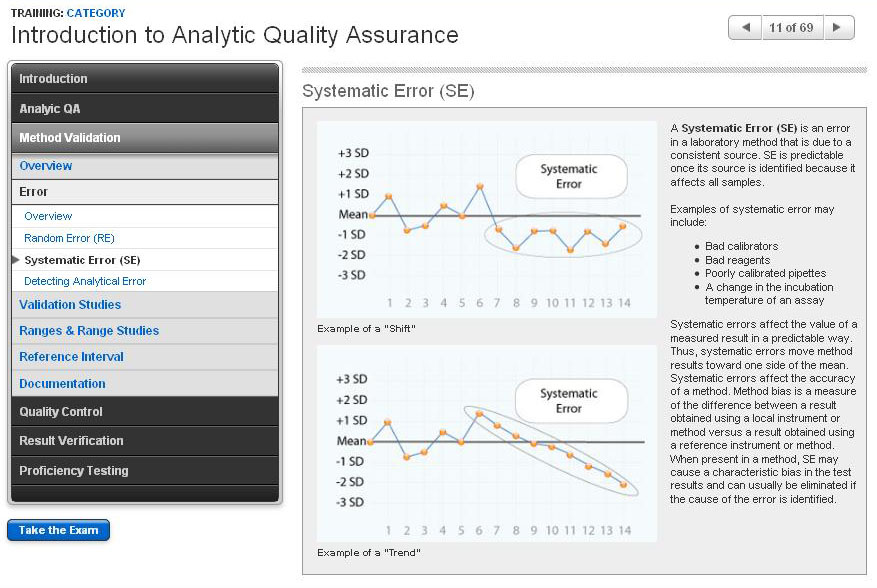
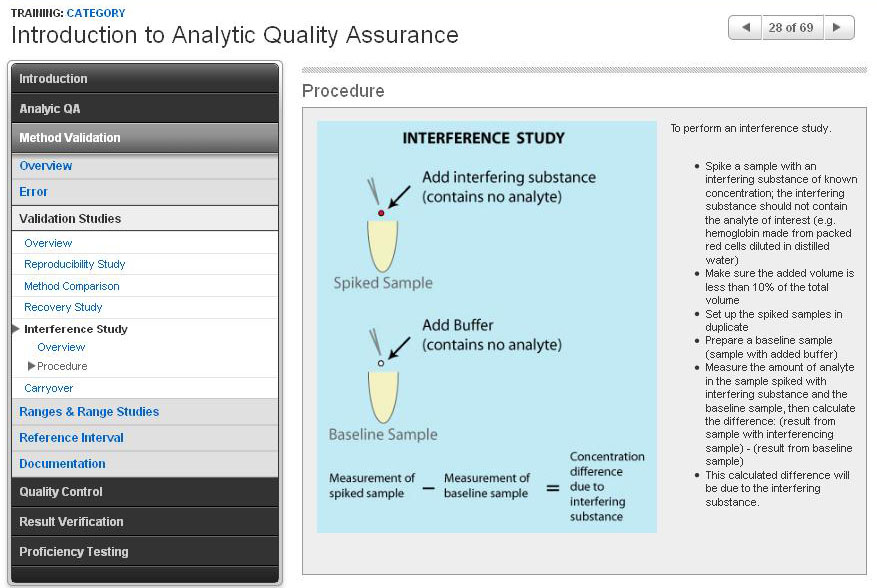
Introduction to Analytic Quality Assurance
Instructor:
Cynthia J. Wilcock, MS, C(ASCP); Timothy Amukele, MD, PhD
Contact Hours:
2.0
Date:
January 01, 2025 - December 31, 2026
Description:
An introduction to analytic quality assurance, quality control, and related topics, including statistical formulas, method validation studies, analytical error, Levey-Jennings interpretation, result verification, and proficiency testing.
Learning Objectives:
- Explain the purpose of analytic Quality Assurance and Quality Control programs in the clinical laboratory.
- Describe the relationship of precision and accuracy to total error and the acceptability of a measurement.
- List at least two causes each of random error and systematic error in a test method.
- Given the mean and standard deviation of a method, calculate the Coefficient of Variation and the Control Range.
- nterpret a Levey-Jennings chart, identifying Shifts, Trends, and other multirule violations.
- Discuss the purpose of Proficiency Testing, describe how these samples are handled in the clinical lab, and how the acceptability of results is determined.